Lithium-ion batteries are essential to today’s technology and can be found in nearly every portable device, as well as electric vehicles. Smartphones, laptops, tablets, cordless power tools, and grid-energy storage are just a few examples that are powered by the excellent battery, which have been a mainstay of electronics since their commercialization back in 1991. The battery’s popularity is due in part to their ability to pack a significant amount of energy into a relatively small space with low weight.
Example of Lithium-ion battery- Nokia BLC-2. (Image credit: Kristoferb via Wikipedia)
Like most batteries, the lithium-ion version offers the same components inside the cell to produce power from a chemical reaction—a positive electrode, a negative electrode, and an electrolyte. In the most widely used in Li-ion technology (for mobile devices) for example, the positive electrode is made of lithium-iron phosphate (LiFePO4), the negative is typically made of carbon (graphite), and the electrolyte is usually comprised of lithium salt in an organic solvent.
Like all technology, the lithium-ion battery has evolved over few decades, incorporating new chemistries for different applications and increased performance. In this roundup, we will look at some of the latest chemistries and compositions that have been developed for lithium-ion batteries and their applications.
Lithium Cobalt Oxide
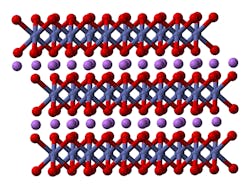
Ball and stick model of part of the crystal structure of lithium cobalt oxide. (Image credit: Ben Mills via Wikipedia)
Lithium cobalt oxide (LiCoO22) batteries are made from lithium carbonate and cobalt and feature very stable capacities along with high-specific energy, making them a popular choice for use with mobile devices such as smartphones, laptops, and digital cameras.
Internally, they are composed of a cobalt oxide cathode and a carbon graphite anode. During discharge, lithium ions travel from anode to cathode, and the process reverses during the recharging cycle. These batteries do have some drawbacks, including a relatively short life cycle, low thermal stability, and smaller load capabilities—meaning they need frequent recharging.
Lithium Manganese Oxide
Panasonic’s CR 2 lithium manganese oxide battery. (Image credit: Kalabaha1969 via Wikipedia)
Lithium manganese oxide (MnO2) batteries actually come in two versions- one with a spinel structure (LiMn2O4), which features a cathode 3D framework for the insertion and desertion of Li-ions during the charge and discharge cycle of the battery. The other comes in layered rock-salt structure (Li2MnO3) with alternating layers of lithium-ions and lithium/manganese ions on the cathode.
Both offer fast charging and high-current discharging with increased thermal stability over cobalt oxide batteries and provide enhanced safety as a result, making them ideal for medical devices, electric vehicles, and power tools.
Lithium Iron Phosphate
Advanced Mobile Power Systems’ Lithium Iron Phosphate 12V battery. (Image credit: AMPS)
Lithium iron phosphate (LiFePO4) batteries use the iron phosphate for the cathode along with a graphite electrode combined with metallic current collector grid for the anode. These batteries are more tolerant at full-charge conditions and are less prone to stress than other Li-ion batteries when subjected to prolonged high voltages.
As a result, these types benefit from low-resistant properties, thereby increasing their safety and thermal abilities, making them ideal for electric motorcycles and vehicles. The only drawback is their low-voltage capacities and offers less energy than other types of Li-ion batteries.
Lithium Nickel Manganese Cobalt Oxide
Melsen Lithium Nickel Manganese Cobalt Oxide battery. (Image credit: Melsen Power)
Lithium nickel manganese cobalt oxide (LiNiMnCoO2) batteries are made using several different elements commonly found in other Li-ion batteries and use a combination of nickel, manganese, and cobalt for the cathode. While the exact material ratios vary by manufacturer, the common combinations are usually 60% nickel, 20% manganese, and 20% cobalt.
Like the other types, these batteries can have either high-specific energy or high-specific power (not both), but the inclusion of nickel provides the cell with the high-specific energy, although it also has reduced stability.
Manganese, on the other hand, provides low internal resistance but has the drawback of low specific energy. Combining the two, however, enhances each other’s strengths, making them suitable for EV powertrains and cordless power tools.
Lithium Nickel Cobalt Aluminum Oxide
Panasonic NCR 18650BM LiNiCoAlO2 batteries. (Image credit: FastTech)
Lithium nickel cobalt aluminum oxide (LiNiCoAlO2) batteries are not conventional in the consumer industry but have promise for EV manufacturers (and other specialized applications), as they provide high-specific energy options, reasonably good specific power, and a decent lifespan.
These types are not as safe as the others listed here and as such, require special safety monitoring measures to be employed for use in EVs. They are also more costly to manufacture, limiting their viability for use in other applications.
Lithium Titanate
Toshiba’s Titanate SCiB (Super Charge ion Battery). (Image credit: Toshiba)
Lithium titanate (LTO) batteries replace the graphite in the anode with lithium-titanate nanocrystals, giving it a larger surface area over carbon, allowing the electrons to enter and exit the anode very quickly. This, in turn, makes it one of the more faster-charging batteries in the Li-ion category.
They do have their disadvantages, however, as they have lower inherent voltage and lower specific-energy ratings over conventional lithium technologies. That being said, they are one of the safer platforms regarding thermal tolerances, making them incredibly safe for use in EVs and e-bikes with possibilities in the military and aerospace industries.
The lithium-ion battery industry is continually evolving with a myriad of types that can be matched for any number of applications. The technology is continuously being improved upon: University of Warwick researchers have developed new technology that allows conventional Li-ion batteries to charge five times faster, while SolidEnergy Systems has developed a more powerful battery using a lithium-metal foil and a proprietary electrolyte that’s purported to have double the energy density of standard Li-ion platforms.
Increased energy capacities, faster recharge time, and enhanced safety, are just the tip of the iceberg for new types Li-ion batteries; we can expect further developments as the latest technology is applied to their development. It will be interesting to see where this time-tested and tried storage medium is headed in the near future.