Energy storage systems employ technological approaches that provide sustainable power for a utility’s customers. These systems must provide extended service during power outages, be easily replenished, be safe, reliable and cost effective. One approach for energy storage involves cross-disciplinary applications in chemistry and electrical engineering. In fact, much of the R&D for electrochemical energy storage is more chemical than electrical. There are several other non-chemical approaches for energy storage systems, but the electrochemical approach is now the dominant solution.
From an electrical standpoint, energy storage systems rectify the incoming ac and convert it to dc for storage in a battery. Then, the stored dc is applied to an inverter to produce an ac output that supplies utility power. These batteries also require some means for recharging them. All electrical functions depend on the characteristics of the batteries and their chemistry.
The chemical part of storage technology involves battery construction. One electrochemical battery is the redox flow battery (RFB) that refers to chemical reduction and oxidation reactions employed to store energy. They employ liquid electrolyte solutions that flow through a battery of cells during charge and discharge.
You can divide RFBs into two categories:
1. True redox flow batteries, where all of the chemical species active in storing energy are fully dissolved in solution at all times.
2. Hybrid redox flow batteries, where at least one chemical is plated as a solid in the electrochemical cells during charge.
True redox flow batteries have two chemical components dissolved in liquids contained within the system and separated by a membrane. Ion exchange through the membrane produces a flow of electric current while both liquids circulate in their own respective space. Cell voltage ranges, in practical applications, from 1.0 to 2.2 V. Generally, stacks of these batteries are used to store utility power.
An example of an RFB is ViZn Energy Systems Inc.‘s (Austin, Texas) Vanguard II battery stack that is immune to cycle-life degradation, providing more headroom to handle spikes in power requirements for demanding and unpredictable applications on both sides of the meter. All ViZn flow batteries incorporate the Vanguard II stack-control technology, which eliminates life-limiting issues such as dendrite growth, simplifies cell balancing, and removes thermal and electrolyte breakdown issues associated with high-frequency power switching.
This unique multi-use capability is necessary for frequency regulation and other high-power applications while adding value to longer duration storage. All of ViZn’s systems are scalable, adding value for even the largest utility requirements. By interconnecting multiple units, both power and energy capabilities can be increased to offer utilities, as well as commercial and industrial customers, the optimal fit for any size project.
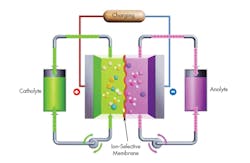
ViZn’s systems utilize an inherently safe, non-toxic, non-explosive zinc-iron electrolyte as shown in the simplified system in Fig. 1. On charging, the electrical energy supplied causes a chemical reduction reaction in one electrolyte and an oxidation reaction in the other. The ion-selective membrane between the half-cells prevents the electrolytes from mixing, but allows selected ions to pass through to complete the redox reaction. On discharge, the chemical energy contained in the electrolyte is released in the reverse reaction and electrical energy can be drawn from the electrodes. When in use, the electrolytes are continuously pumped in a circuit between reactor and storage tanks.
High-power flow batteries use multiple stacks of cells. The size and number of electrodes in the cell stacks is fixed and determines the system’s power rating. An advantage of this system is that it provides electrical storage capacity, limited by the capacity of the electrolyte storage reservoirs. Facilitating thermal management is use of the electrolytes as the thermal working fluids as they are pumped through the cells.
ViZn’s zinc-iron Redox storage technology provides large-scale energy storage. A modular unit is a 20- or 40-ft. shipping container that can be combined and scaled to provide storage solutions for projects ranging from 100 kW to 100 MW. This technology provides safety, simplicity, and use of abundant core non-toxic raw materials to achieve a 20-year expected life. Figure 2 shows its internal construction.
One example of a hybrid redox flow battery is the all-iron redox flow battery (IFB) developed by ESS (Portland, Ore.). The IFB is a durable, environmentally safe, long-duration storage solution. Its lifespan that exceeds 20,000 cycles, it has low maintenance requirements, and an energy capacity of over six hours. The IFB matches well with the 25-year life span of solar and wind projects, supporting those applications’ low levelized cost of energy (LCOE) requirements. In addition, the IFB’s inherent quick-response power electronics can perform ancillary services such as voltage and frequency support on microgrids and utility-scale applications.
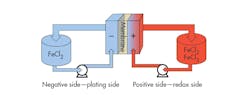
The IFB’s all-iron redox flow battery technology uses iron dissolved in salt water as an electrolyte (Fig. 3). You can break down IFB performance to its plating electrode performance (negative electrode), redox electrode performance (positive electrode), and ohmic resistance loss. On the plating electrode, the ferrous (Fe2+) ion gains electrons and plates as solid iron on the substrates during charge, and the solid iron dissolves as ferrous ions and releases two electrons during discharge. The equilibrium potential for the iron plating reaction is -0.44V. On the redox electrode, the redox reaction between ferrous and ferric (Fe3+) ions occurs during charge and discharge. On the positive electrode, two Fe2+ ions lose two electrons to form Fe3+ ions during charge and two Fe3+ ions gain two electrons to form Fe2+ during discharge. The equilibrium potential between ferrous and ferric ions is +0.77V. Thus, the reaction in an IFB redox flow battery is reversible.
A recent report by IDTechEx Research, authored by technology analyst Dr. Lorenzo Grande covered Redox Flow Batteries 2017-2027: Markets, Trends, Applications. The report noted that RFBs retain most of their initial value thanks to the possibility to recycle their core components more easily than other battery chemistries. It also predicted that the RFB market will be worth $4B by 2027 and will include all stationary storage applications, from residential to commercial and industrial to grid-scale systems. It said RFBs can potentially make second-life li-ion batteries obsolete by offering a stable cycle life and reduced engineering and battery management system challenges. Recycling aspects will ensure that, once out of service, the raw materials will retain most of their value and will be used in brand new RFBs.
Liquid Metal Batteries
Ambri, an MIT spinoff (Cambridge, Mass.), developed a liquid metal battery made of magnesium and antimony, but the metals had to be melted into liquids by getting heated to 700°C (about 1,300°F). Today, a new and improved molten metal battery operates at only 900°F. They don’t use magnesium and antimony now, but something similar.
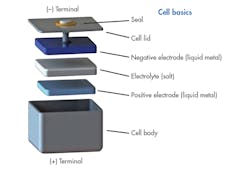
The battery is contained in liquid metal storage cells that look like stainless-steel shoe boxes (Fig. 4). Boxes are filled with raw materials and vacuum sealed. The production process uses low-cost capital equipment with high-capacity throughput that allows them to scale battery size. It’s a simple technology with no moving parts and no separators between the layers as employed in redox batteries.
The electrochemistry of the battery involves three liquid components that establish a potential that produces a flow of electricity. It consists of:
1. Liquid metal as the bottom positive electrode, which pools on the bottom of a stainless-steel can.
2. Layer of molten salt, which floats on top of that.
3. A second metal that floats on top of the salt.
On discharge, the top electrode metal dissolves into the electrolyte as an ion, shedding electrons that go through an external circuit delivering power. Reverse the flow and the electrons return to the positive electrode, charging the battery. The molten metals have different densities and don’t mix. Like oil and water, they separate into layers: no pumps, no moving parts.
The system essentially runs itself. A beta prototype battery has 432 storage cells strung together and placed into a small insulated shipping container (Fig. 5). A commercial version could supply a day’s worth of electricity to 30 average Massachusetts homes. To scale up: add more containers.
Inside the box it’s 900 degrees but outside it’s cool to the touch. The system is about 80% efficient, with very little energy wasted. The inefficiency is given off as heat that is held within the insulated box that holds the cells. That is sufficient to keep it at operating temperature, and you just charge and discharge to keep it in that state.
Ambri built a beta prototype, 432 storage cell battery housed in a small insulated shipping container. A commercial version of this 20kW-hour battery could supply a day’s worth of electricity to 30 average homes.
Li-ion Battery Energy Storage
You may not consider Li-ion batteries as employing electrochemistry, but they do. They are definitely a candidate for energy storage. Li-ion batteries that have seen cost declines due to the rapid rise in electric vehicle production and sales. Industry sources say that Li-ion batteries sold for EVs have gone from 250 MWh in 2010 to more than 52,000 MWh in 2016. Large-format Li-ion battery pack costs have gone from $1,000/kWh in 2010 to $350 in 2015. If the cost of Li-ion batteries continues to decline faster than that of the other battery technologies, the cost difference may overwhelm the operational advantages of other energy storage technologies.
The Energy Storage Association says Li-ion technologies have widely differing life and safety characteristics. Cells with positive materials based on lithium iron phosphate are inherently safer than their metal oxide/carbon counterparts but the voltage is lower (around 3.2 V), as is the energy density. Designs with lithiated metal oxide positives and lithium titanate negatives have the lowest voltage (around 2.5 V) and low energy density but have much higher power capability and safety advantages.
You can produce Li-ion cells in cylindrical or prismatic (rectangular) format. These cells are then typically built into multi-cell modules in series/parallel arrays, and the modules are connected together to form a battery string at the required voltage, with each string being controlled by a battery management system. Electronic subsystems that protect Li-ion batteries are important assets. Safety characteristics of Li-ion batteries are ultimately determined by the attributes of system design, including mechanical and thermal characteristics, electronics and communications, and control algorithms, regardless of electrochemistry.
Energy Storage Research
MIT’s energy storage center will draw on cross-disciplinary research in engineering, science, and policy as well as real-world input from stakeholders in industry, government, and non-governmental organizations to hasten the development of new energy storage technologies with the technical performance and cost characteristics needed to provide power sustainably at any place, at any scale, and at any time. The center is led by co-directors Jeffrey Grossman, the Morton and Claire Goulder and Family Professor in Environmental Systems and a professor of materials science and engineering at MIT, and Yang Shao-Horn, the W.M. Keck, Professor of Energy and a professor of mechanical engineering and of materials science and engineering at MIT.
The research portfolio at the center will mirror the wide variety of energy storage needs that must be addressed to enable greater deployment of renewables in the power sector and more extensive electrification of mobility. Examples include developing new lithium-ion and sodium-ion battery materials with increased storage capacity and fuels that can store solar energy as usable, distributable, on-demand chemical energy.
In addition, researchers are investigating ways to control, synthesize, and characterize materials at the atomic and nanometer scales—work that will facilitate the discovery and design of new materials for storage applications.
Researchers from Harvard University’s John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed a new flow battery that stores energy in organic molecules dissolved in neutral pH water. This new chemistry allows a non-toxic, non-corrosive battery with an exceptionally long lifetime and offers the potential to significantly decrease production costs.
The research was led by Michael Aziz, the Gene and Tracy Sykes Professor of Materials and Energy Technologies and Roy Gordon, the Thomas Dudley Cabot Professor of Chemistry and Professor of Materials Science.
By modifying the structures of molecules used in the positive and negative electrolyte solutions, and making them water soluble, the Harvard team was able to engineer a battery that loses only 1% of its capacity per 1,000 cycles. “Lithium ion batteries don’t even survive 1,000 complete charge/discharge cycles,” said Aziz.
This reduction of cost is important. The Department of Energy (DOE) has set a goal of building a battery that can store energy for less than $100 per kilowatt-hour, which would make stored wind and solar energy competitive with energy produced from traditional power plants.
Research was supported by the Office of Electricity Delivery and Energy Reliability of the DOE (Department of Energy) and by the DOE’s Advanced Research Projects Agency-Energy.