A new combination of materials developed by Stanford University researchers may aid in developing a rechargeable battery that can store the large amounts of renewable power created through wind or solar sources. With further development, the new technology could deliver energy to the electric grid quickly, cost effectively, and at normal ambient temperatures.
A flow battery has long been considered as a likely candidate for storing intermittent renewable energy. However, the kinds of liquids that could produce the electrical current have either been limited by the amount of energy they could deliver, required extremely high temperatures, or used very toxic or expensive chemicals.
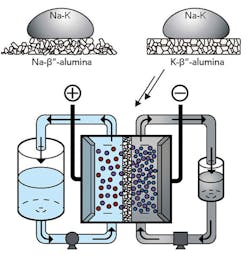
Stanford assistant professor of materials science and engineering William Chueh, along with his PhD student Antonio Baclig and Jason Rugolo, now a technology prospector at Alphabet’s research subsidiary X Development, decided to try sodium and potassium as the fluid for the electron donor—or negative—side of the battery. When mixed, the materials form a liquid metal at room temperature. Theoretically, this liquid metal has at least 10 times the available energy per gram as other candidates for the negative-side fluid of a flow battery. The figure shows the prototype flow battery system.
Sodium-potassium alloy is a room-temperature liquid metal that could unlock a high-voltage flow battery. (Credit: Antonio Baclig)
“We still have a lot of work to do,” says Baclig, “but this is a new type of flow battery that could affordably enable much higher use of solar and wind power using Earth-abundant materials.”
In order to use the liquid metal negative end of the battery, the group found a suitable ceramic membrane made of potassium and aluminum oxide to keep the negative and positive materials separate while allowing current to flow.
The two advances together more than doubled the maximum voltage of conventional flow batteries, and the prototype remained stable for thousands of hours of operation. This higher voltage means the battery can store more energy for its size, and thus brings down the cost of producing the battery.
“A new battery technology has so many different performance metrics to meet: cost, efficiency, size, lifetime, safety, etc.,” says Baclig. “We think this sort of technology has the possibility, with more work, to meet them all, which is why we are excited about it.”
The team of Stanford PhD students, which in addition to Baclig includes Geoff McConohy and Andrey Poletayev, found that the ceramic membrane very selectively prevents sodium from migrating to the positive side of the cell—a critical factor if the membrane is to succeed. However, this type of membrane is most effective at temperatures higher than 200°C (392°F). In pursuit of a room-temperature battery, the group experimented with a thinner membrane. This boosted the device’s power output and showed that refining the membrane’s design is a promising path.
They also experimented with four different liquids for the positive side of the battery. The water-based liquids quickly degraded the membrane, but they think a non-water-based option will improve the battery’s performance.
This project was funded by Stanford’s TomKat Center for Sustainable Energy, the Anthropocene Institute, the State Grid Corporation of China through Stanford’s Energy 3.0 corporate affiliate program, the National Research Foundation of Korea, the U.S. National Science Foundation, and Stanford Graduate Fellowships.
Liquid Battery
In a paper published in the journal Nature Chemistry, chemists from the University of Glasgow discuss how they developed a flow battery system using a nano-molecule that can store electric power or hydrogen gas. It, in turn, presents a new type of hybrid energy-storage system that can be used as a flow battery or for hydrogen storage.
Their “hybrid-electric-hydrogen” flow battery, based on the design of a nanoscale battery molecule, can store energy, releasing the power on demand as electric power or hydrogen gas that can be used a fuel. When a concentrated liquid containing the nano-molecules is made, the amount of energy it can store increases by almost 10 times. The energy can be released as either electricity or hydrogen gas, meaning that the system could be used flexibly in situations that might need either a fuel or electric power.
One potential benefit of this system is that electric cars could be charged in seconds, since the material is a pumpable liquid. As a result, the battery of an electric car could perhaps be “recharged” in roughly the same length of time as petrol cars can be filled up. The old battery liquid would be removed at the same time and recharged ready to be used again.
The approach was designed and developed by Professor Leroy (Lee) Cronin, the University of Glasgow’s Regius Chair of Chemistry, and Dr Mark Symes, Senior Lecturer in Electrochemistry, also at the University of Glasgow with Dr Jia Jia Chen, who is a researcher on the team. They’re convinced that this result will help pave the way for the development of new energy-storage systems that could be used in electric cars, for the storage of renewable energy, and to develop electric-to-gas energy systems for scenarios when a fuel is required.
Professor Cronin says, “For future renewables to be effective, high-capacity and flexible energy-storage systems are needed to smooth out the peaks and troughs in supply. Our approach will provide a new route to do this electrochemically and could even have application in electric cars, where batteries can still take hours to recharge and have limited capacity. Moreover, the very high energy density of our material could increase the range of electric cars, and also increase the resilience of energy-storage systems to keep the lights on at times of peak demand.”
This research is funded by the University of Glasgow complex chemistry initiative as well as the European Research Council (ERC) and the Engineering and Physical Sciences Research Council (EPSRC).